Harnessing proteomics
Dr. Birgit Schilling, a trailblazer from the Buck Institute for Research on Aging, studies proteomics to unveil the mysteries of aging-related diseases. In this video, Birgit shares her innovative journey using the SCIEX ZenoTOF 7600 system to explore acute kidney injury in mice and dive into the complexities of human breast cancer.
Extraordinary science with Birgit Schilling
The Buck Institute for Research on Aging studies proteomics to unveil the mysteries of aging-related diseases

Unlocking the secrets of aging-related diseases
A deep dive into kidney proteome remodeling
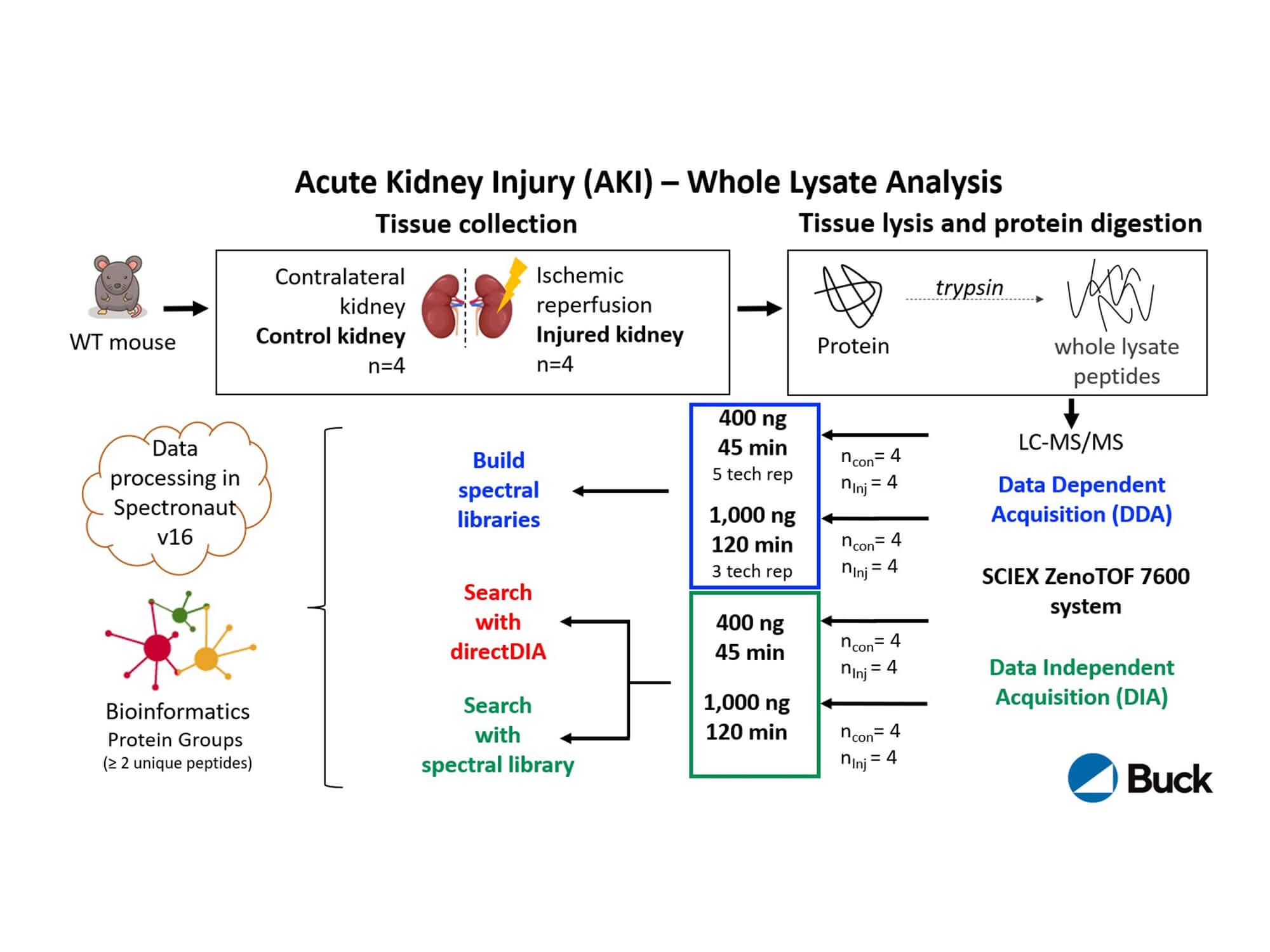
In this renal study, Birgit Schilling explores the transformations that occur in the kidney proteome during acute kidney injury. Her research reveals that in response to injury, the kidney proteome was completely remodeled and more than half of the 3,945 quantified protein groups were significantly changed. Notably, many downregulated proteins in the injured kidney were involved in energy production. Numerous peroxisomal matrix proteins related to lipid metabolism were also downregulated, including ACOX1, CAT, EHHADH, ACOT4, ACOT8 and Scp2. The sensitive kidney-specific SWATH data-independent acquisition (DIA) assays that were performed allowed for high-throughput coverage of the kidney proteome, promising a new path toward innovative therapies to restore kidney function.
Revolutionizing breast cancer research
For her breast cancer project, Birgit Schilling developed a novel FFPE-deparaffinization protocol in combination with SWATH DIA assays that enabled the quantitation of up to 6,000 proteins. Her research reveals that many small leucine-rich, desmosome and basement membrane proteins that contribute to the stiffness of the extracellular matrix (ECM) microenvironment were significantly down-regulated. The reduced proteins included HSPG2, desmoplakin, lumican and many collagens, whereas Col12A1 was upregulated. Additionally, the results showed that 74 quantified senescence-associated secretory phenotype proteins (SASP factors) were significantly altered when compared to healthy tissue. Notably, SerpinH1 and periostin were up-regulated in all breast cancer subtypes. SerpinH1, for example, has previously been validated as a significant biomarker and cancer-associated protein in lung cancer. These findings demonstrate that changes in the ECM and SASP factors are observed in breast cancer progression, pointing towards potential therapeutic strategies targeting these pathways.
Key takeaways
- Comprehensive SWATH DIA with 80 variable windows deciphers disease mechanisms and disease pathways
- Short microflow gradients accelerate quantitation workflows while maintaining quantitation accuracy in mammalian tissues
- Complete remodeling of the kidney proteome upon acute kidney injury
- Optimized FFPE tissue analysis using SWATH DIA (with up to 6,000 proteins quantified) highlights breast cancer biology and cancer progression in 5 different breast cancer subtypes
Watch Birgit Schilling’s extraordinary science
About the presenter

Birgit Schilling, PhD
Director of the Mass Spectrometry Core
Schilling Laboratory
The Buck Institute for Research on Aging
Birgit Schilling has worked at the Buck Institute for Research on Aging in the San Francisco Bay Area since 2000 as Professor and Director of the Mass Spectrometry Technology Center. Her work focuses on data-independent acquisition technologies and large-scale proteome quantitation. Birgit received her PhD in Germany and then moved to the University of California San Francisco (UCSF) as a postdoctoral fellow. She is interested in translational research that can support the development of therapeutic interventions to improve human aging or age-related diseases, including osteoarthritis, kidney injury and cancer. Birgit uses modern proteomic technologies to investigate mechanisms of aging, senescence and cancer. She uses this knowledge to develop biomarkers and targets for interventions.
Related publications
-
Localization and quantification of post-translational modifications of proteins using electron activated dissociation fragmentation on a fast-acquisition time-of-flight mass spectrometer
-
Substantial downregulation of mitochondrial and peroxisomal proteins during acute kidney injury revealed by data-independent acquisition proteomics [Preprint]