Rapid detection and identification of 105 performance-enhancing substances in animal-derived foods
Yang Zong1, Chen Dan2, Cheng Haiyan1, Li Lijun1 and Guo Lihai1
1SCIEX Asia Pacific Applicaton Support Centre, China; 2Wuhan Institute for Food and Cosmetic Control, China
Abstract
In this technical note, a comprehensive screening method for the analysis of 105 common performance-enhancing substances in animal-derived foods was developed. Using the SCIEX QTRAP 4500 system, multiple reaction monitoring (MRM)-trigged enhanced product ion (EPI) scans allowed for the simultaneous acquisition of highly sensitive MRM quantitation data and MS/MS spectra for compound confirmation using library searching (Figure 1). The foods evaluated included milk, eggs and lean meat. The sample preparation procedure was optimized by testing various extraction solvents and solid-phase extraction (SPE) cartridges to maximize analyte recovery. Additionally, various LC columns were evaluated to optimize analyte retention, peak shape and chromatographic resolution of this diverse compound panel.
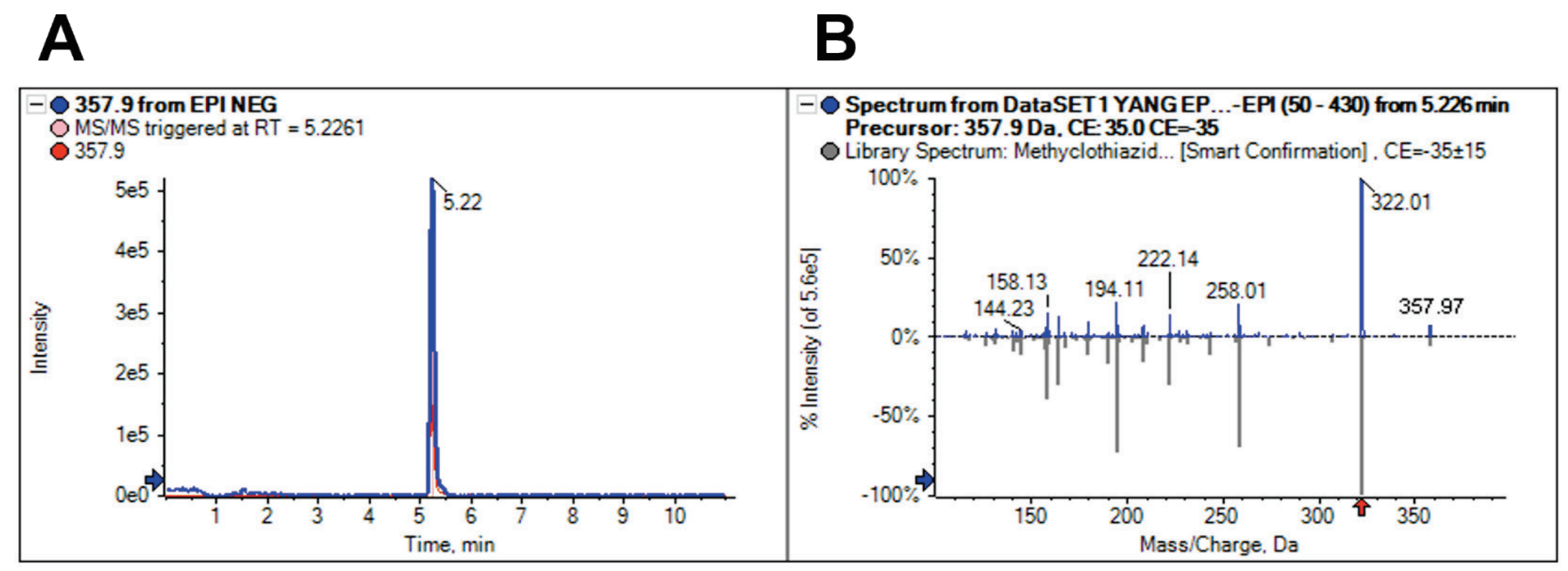
Figure 1. Accurate identification of methyclothiazide in milk. A) Extracted ion chromatogram (XIC) and B) full MS/MS spectrum showing confident identification of methyclothiazide in a milk sample. The MRM XIC provided quantitative data and the full scan MS/MS spectrum was matched to the library spectrum for confident identification of performance-enhancing substances in animal-derived food samples.
Key benefits for the analysis of performance-enhancing substances in animal-derived food using the QTRAP 4500 system
- Wide analyte coverage: The method covered a wide range of performance-enhancing substances, including 5 common types of β-receptor agonists, steroid hormones, glucocorticoids, diuretics and stimulants
- Broad matrix applicability: Sample preparation procedures were compatible with diverse animal-derived food matrices (milk, egg and lean meat)
- Optimized sample preparation: Acidified acetonitrile provided the best recovery values across the 3 different matrices
- Simultaneous quantitative and qualitative analyses: Highly sensitive and selective Multiple Reaction Monitoring (MRM) survey scan, followed by full scan MS/MS analysis (EPI – Enhanced Product Ion scan) enabled both quantitation and compound identification using MS/MS library searching
Introduction
Foodborne doping can originate from the presence of endogenous substances but often arises from artificial, exogenous compounds, such as performance-enhancing substances, that are intentionally added to animal feed to improve its nutritional value.1 These substances are included on the World Anti-Doping Agency (WADA) Prohibited List due to their ability to enhance athletes' overall performance. For example, these substances are commonly used by athletes to reduce fatigue, increase muscle mass and improve strength, endurance, alertness and competitiveness.2 However, they also represent a potential human health risk, as their misuse can potentially result in effects such as a rapid heart rate and high blood pressure. For these reasons, there is a need to actively monitor the presence of performance-enhancing substances in animal-derived foods to ensure fairness and to maintain the integrity of competition, in addition to ensuring the health and safety of the athletes consuming them.
Methods
Target analytes and samples: A panel consisting of 105 performance-enhancing substances, including 5 common types of β-receptor agonists, steroid hormones, glucocorticoids, diuretics and stimulants was selected for this study. Three different animal-derived products were analyzed, including milk, egg and lean meat.
Sample preparation: The 105 performance-enhancing substances were extracted from the 3 animal-derived products using a liquid-liquid extraction (LLE) procedure followed by a SPE step. The full extraction procedure is summarized in Figure 2. Three extraction buffers were tested in step 5 of this procedure to identify the solvents that resulted in the best analyte recoveries. Additionally, 5 different SPE cartridges were evaluated in step 9 to determine the maximum analyte recovery. The specific SPE cartridges tested included the Agela Cleanert PEP-2 SPE column (200 mg/6 mL), Waters Oasis PRiME HLB (6 cc/200 mg), Aladdin Scientific (AR|2.5 kg AR|500 g), Waters Sep-Pack C18 (3 cc Vac) and Agela Cleanert Silica (200 mg/3 mL) cartridges.
Figure 2. The 11-step extraction protocol used to selectively extract performance-enhancing substances from animal-derived products. An LLE procedure was followed by an SPE step to selectively extract analytes from milk, egg and lean meat for analysis with the QTRAP 4500 system.
Liquid chromatography: HPLC separation was performed on an ExionLC system. Three LC columns were tested to determine which provided the best separation and peak shape for the 105 compounds included in the panel. The 3 LC columns used in these experiments included a Waters Acquity UPLC BEN C18, a Hawach ODS C18 and a Phenomenex Kinetex™ F5 (150 × 3.0 mm, 2.6 µm, 00F-4723-Y0) column. Mobile phase A (MPA) and mobile phase B (MPB) were 5mM ammonium acetate with 0.01% formic acid and acetonitrile, respectively. The total LC runtime was 25 minutes and the injection volume was 5 µL.
Mass spectrometry: Analysis was performed on the SCIEX QTRAP 4500 system with an IonDrive Turbo V ion source. The 105 performance-enhancing substances were detected in positive and negative electrospray ionization (ESI) modes with Analyst software, version 1.7 using polarity switching. The scheduled MRM algorithm was used to acquire enough data points to quantify the data. This was followed by a full EPI scan MS/MS analysis on the detected analytes using the linear ion trap mode.
Data processing: Data were processed using SCIEX OS software, version 2.0.1. MS/MS spectral library matching and MS/MS library searching were performed for the confirmation of the detected compounds.
Optimized sample extraction procedure leads to improved analyte recoveries
The ability to reliably detect analytes from the sample matrix relies on the efficiency of the sample extraction procedure. As part of the sample extraction procedure optimization process, 3 extraction buffers were tested to determine the solvent that provided the best analyte recoveries. Figures 3, 4 and 5 show the recovery values achieved for each of the 3 extraction buffers (alkalized acetonitrile, acidified acetonitrile and tert-butyl methyl ester) for several representative analytes extracted from milk, egg and lean meat, respectively. The results demonstrate that using acidified acetonitrile provided the best recovery values across the 3 different animal-derived food matrices. As a result, this extraction buffer was used for the rest of the experiments.
Due to the complexity of the 3 animal-derived products used for these experiments, SPE was performed following the solvent extraction step. Five SPE cartridges were tested to compare their extraction efficiencies. Figure 6 shows the extraction recoveries for 46 representative analytes included in the panel for each of the 5 SPE cartridges. The Waters Oasis PRiME HLB (6 cc/200 mg) cartridge provided the highest recovery rates for the set of analytes analyzed and was chosen as the default cartridge for the rest of the experiments (Figure 6).
Figure 3. Extraction recoveries achieved using the 3 extraction buffers for 28 representative analytes included in the panel and extracted from milk. Acidified acetonitrile provided the best extraction recovery values out of the 3 extraction buffers tested for these experiments.
Figure 4. Extraction recoveries achieved using the 3 extraction buffers for 50 representative analytes included in the panel and extracted from egg. The best extraction recovery values were achieved for the panel of 51 representative analytes when acidified acetonitrile was used as the extraction buffer.
Chromatographic separation of the 105 performance-enhancing substances
Chromatographic separation of a large panel of compounds in a single method is critical to ensure the number of compounds analyzed at a particular time is minimized. Achieving baseline resolution minimizes MRM concurrency for the analytes and allows the balance between cycle time of the instrument and the dwell time for each compound to be optimized. Three LC columns were evaluated for the separation of the 105 compounds included in the panel. The experiment demonstrated that the Phenomenex Kinetex F5 column beter separated the more polar diuretics and β-receptor agonists, in addition to the less polar steroid hormones, compared to either of the other 2 columns. The Phenomenex Kinetex F5 column also beter retained the analytes and provided the sharpest chromatographic peak. Figure 7 shows the extracted ion chromatogram (XIC) for the 105 performance-enhancing substances analyzed in both positive (A) and negative (B) ESI modes. The compounds were spiked in milk at 10 µg/mL, extracted using the developed sample preparation procedure and separated using the Phenomenex Kinetex F5 column. The results demonstrated that the chromatographic conditions used in this workflow yielded separations sufficient to accurately detect each analyte included in the panel.
Figure 5. Extraction recoveries achieved using the 3 extraction buffers for 35 representative analytes included in the panel and extracted from lean meat. The use of acidified acetonitrile as the extraction buffer provided the best recovery values for the panel of 35 representative analytes.
Confident identification of performance-enhancing substances using full scan MS/MS and spectral library searching
The QTRAP 4500 system was used to analyze the food-derived products to enhance confidence in compound identification. By capturing MRM and EPI MS/MS confirmation scans in 1 injection, high-quality MRM quantitation with MS/MS confirmation was obtained. The acquired full scan MS/MS spectra contained the complete fragmentation patern of the compounds and was searched against a mass spectral library. Figure 1 shows the XIC and the acquired MS/MS spectrum with the MS/MS matched library spectrum for the analysis of a milk sample. The acquisition of the MS/MS spectrum enabled positive identification of methyclothiazide, a thiazide diuretic, through spectral library matching. The use of spectral library matching provided increased identification confidence for the confirmation of performance-enhancing substances in animal-derived foods.
Figure 6. Extraction recoveries achieved using the 5 SPE cartridges for 46 representative analytes included in the panel. The Waters Oasis PRiME HLB (6 cc/200 mg) SPE cartridge provided the highest recovery rates for the set of analytes included in the representative analysis.
Figure 7. XICs for the 105 performance-enhancing substances analyzed in both positive (A) and negative (B) ESI modes. The optimized LC conditions enabled baseline separation of the 105 performance-enhancing substances included in the panel. The polarity switching capabilities of the QTRAP 4500 system enabled data acquisition in both positive and negative ion acquisition modes in a single injection.
Conclusion
This technical note demonstrated:
- A comprehensive screening method for detecting and identifying 105 common performance-enhancing substances in animal-derived foods using the QTRAP 4500 system
- An optimized sample preparation procedure comprising solvent extraction and SPE cleanup
- Optimized LC chromatography with good peak shape quality for the panel of 105 analytes in both positive and negative ion acquisition modes within a single injection
- The MRM-triggered EPI scanning function of the QTRAP 4500 system allowed for simultaneous quantitation and confirmation using library searching
References
- Heming, Q.; Shen, H.; Meiling, L.; Yin, Z.; Li, J.; Cui, F. Simultaneous determination of 18 food-borne stimulant drug residues in beef by ultra-high performance liquid chromatography-tandem mass spectrometry. Se Pu., 2018, 36 (7), 621- 628.
- Choi, T.L.S.; Kwok, K.Y.; Kwok, W.H.; Tsoi, Y.K.; Wong, J.K.Y.; Wan, T.S.M. Detection of seventy-two anabolic and androgenic steroids and/or their esters in horse hair using ultra-high performance liquid chromatography-high resolution mass spectrometry in multiplexed targeted MS2 mode and gas chromatography tandem mass spectrometry. J. Chromatogr. A, 2018, 1566 (7), 51-63
 Click to enlarge
Click to enlarge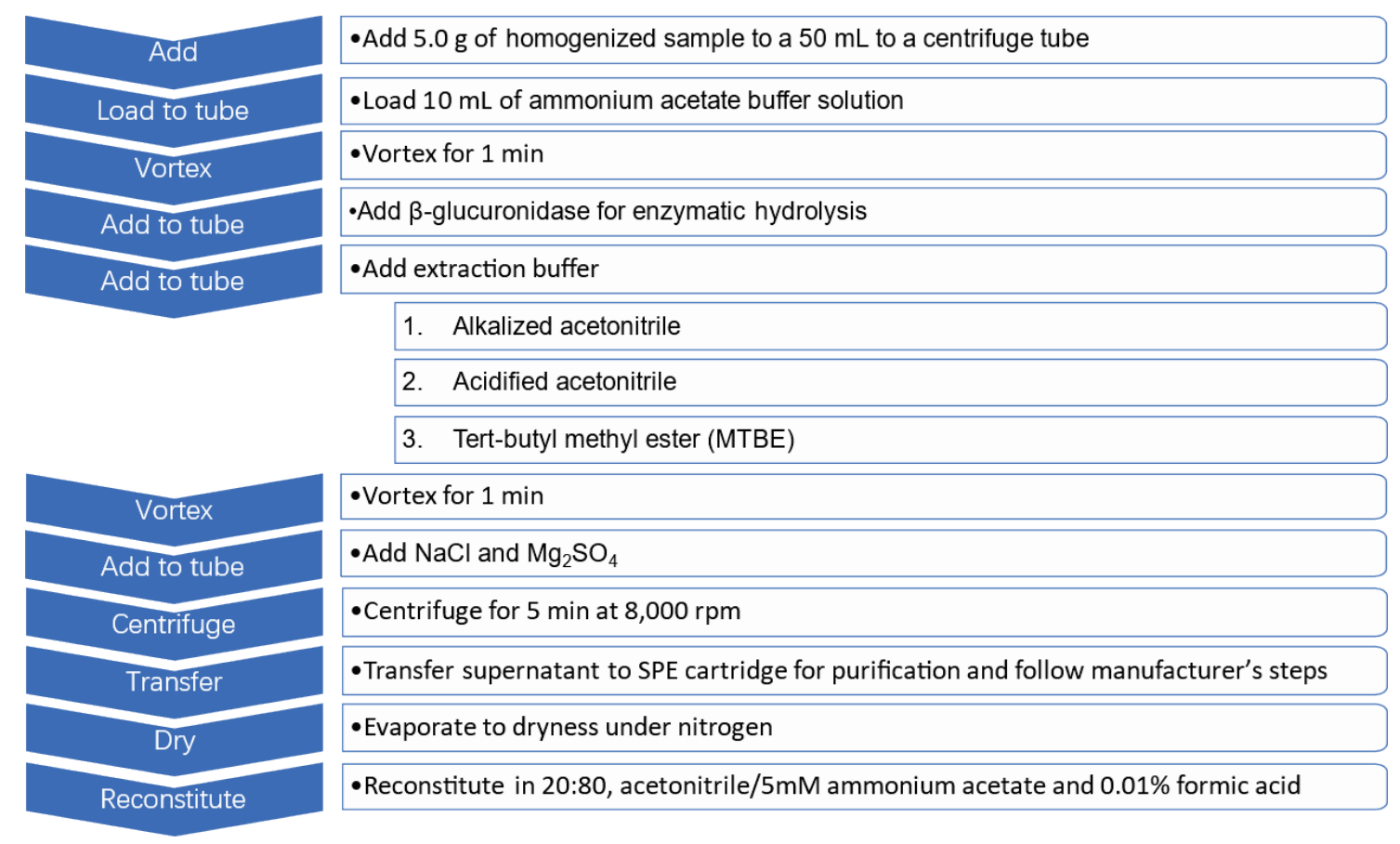 Click to enlarge
Click to enlarge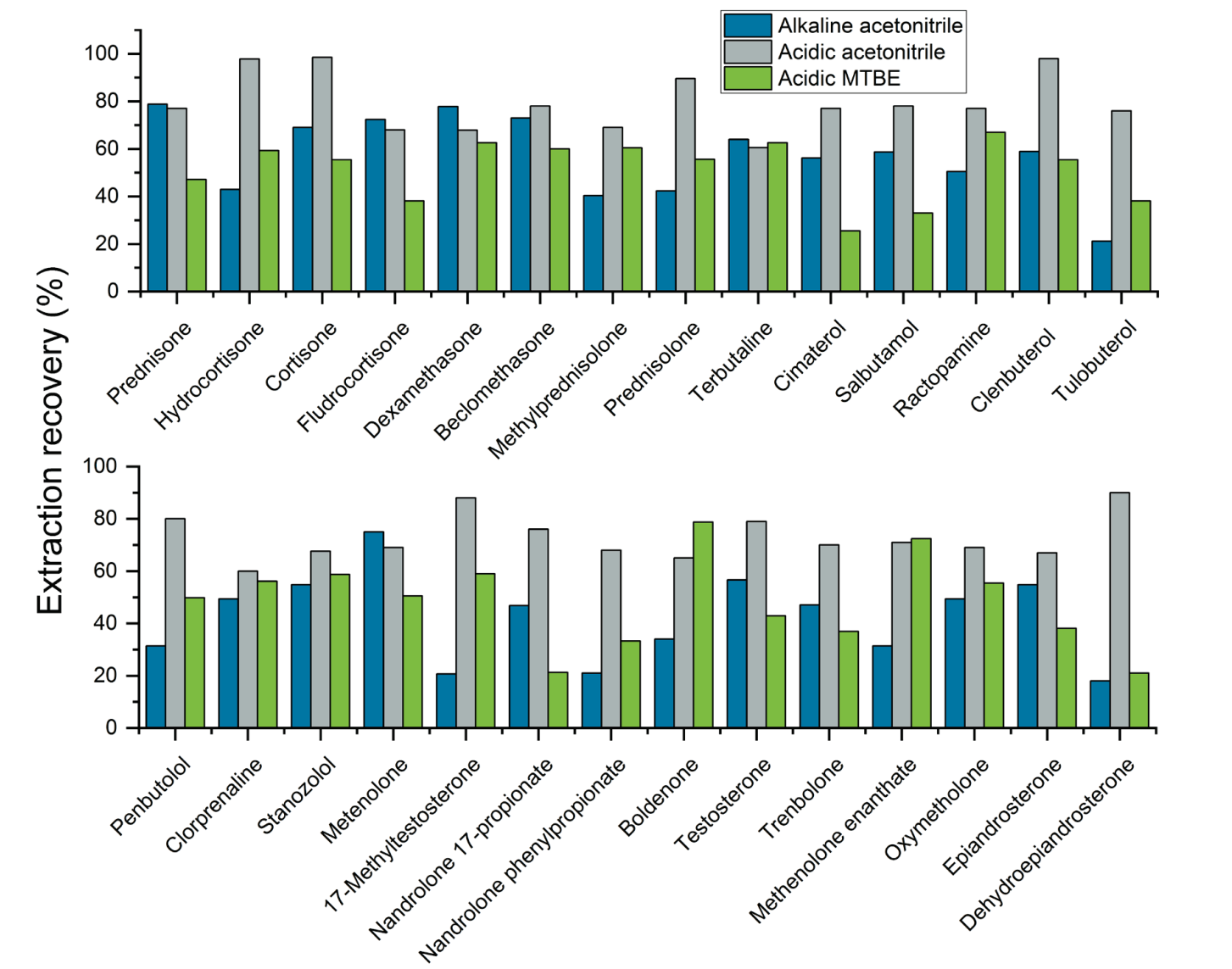 Click to enlarge
Click to enlarge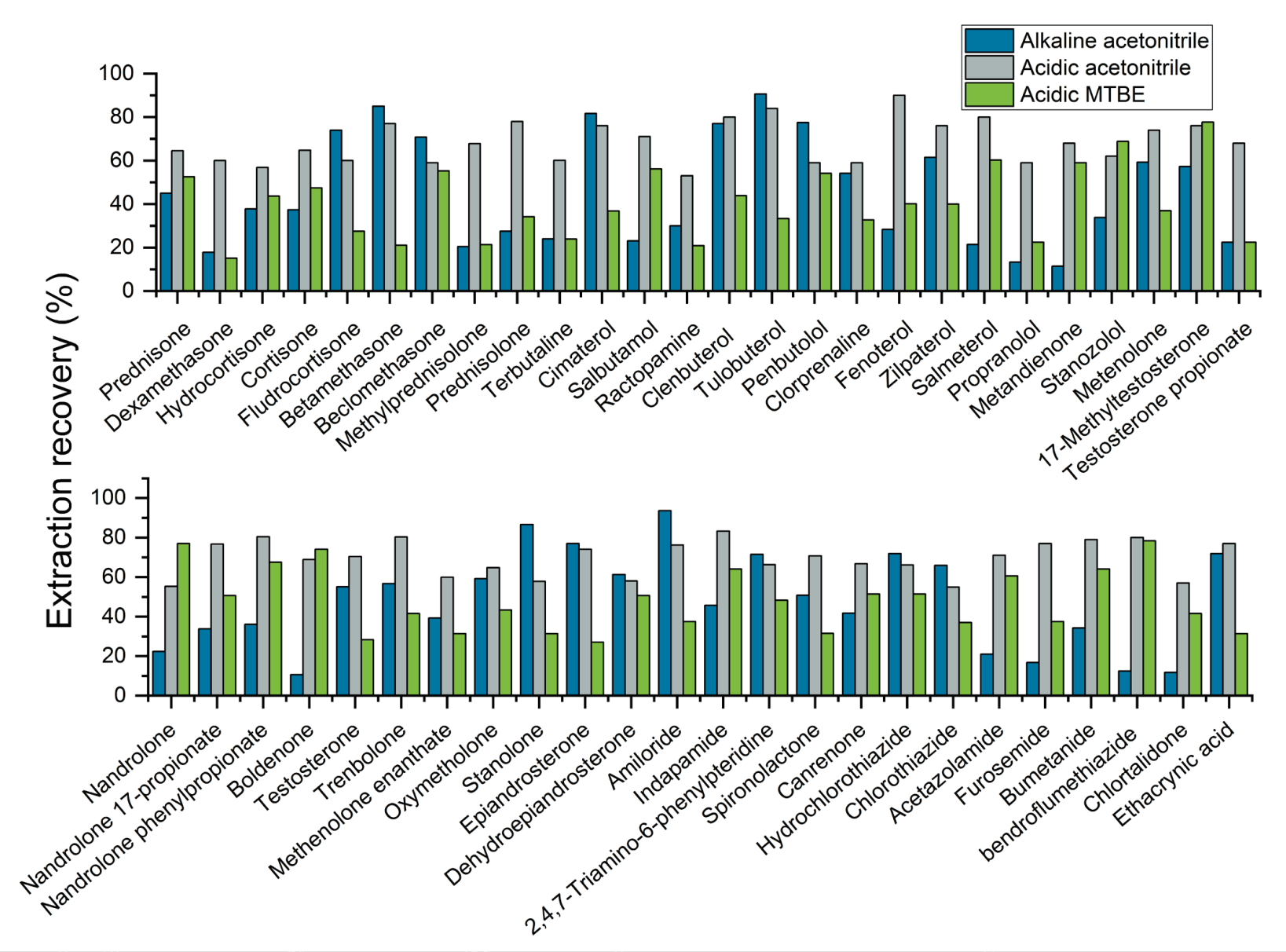 Click to enlarge
Click to enlarge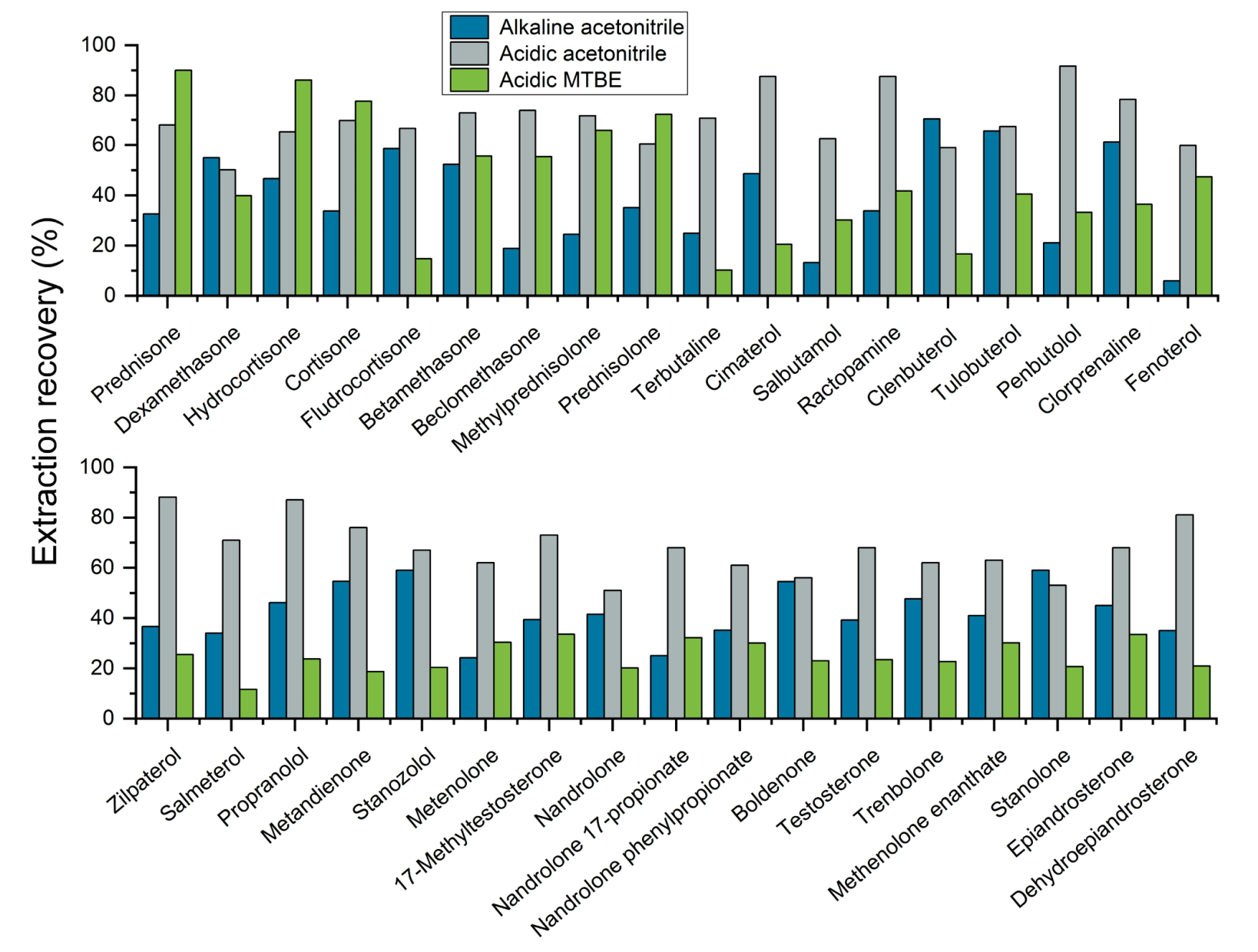 Click to enlarge
Click to enlarge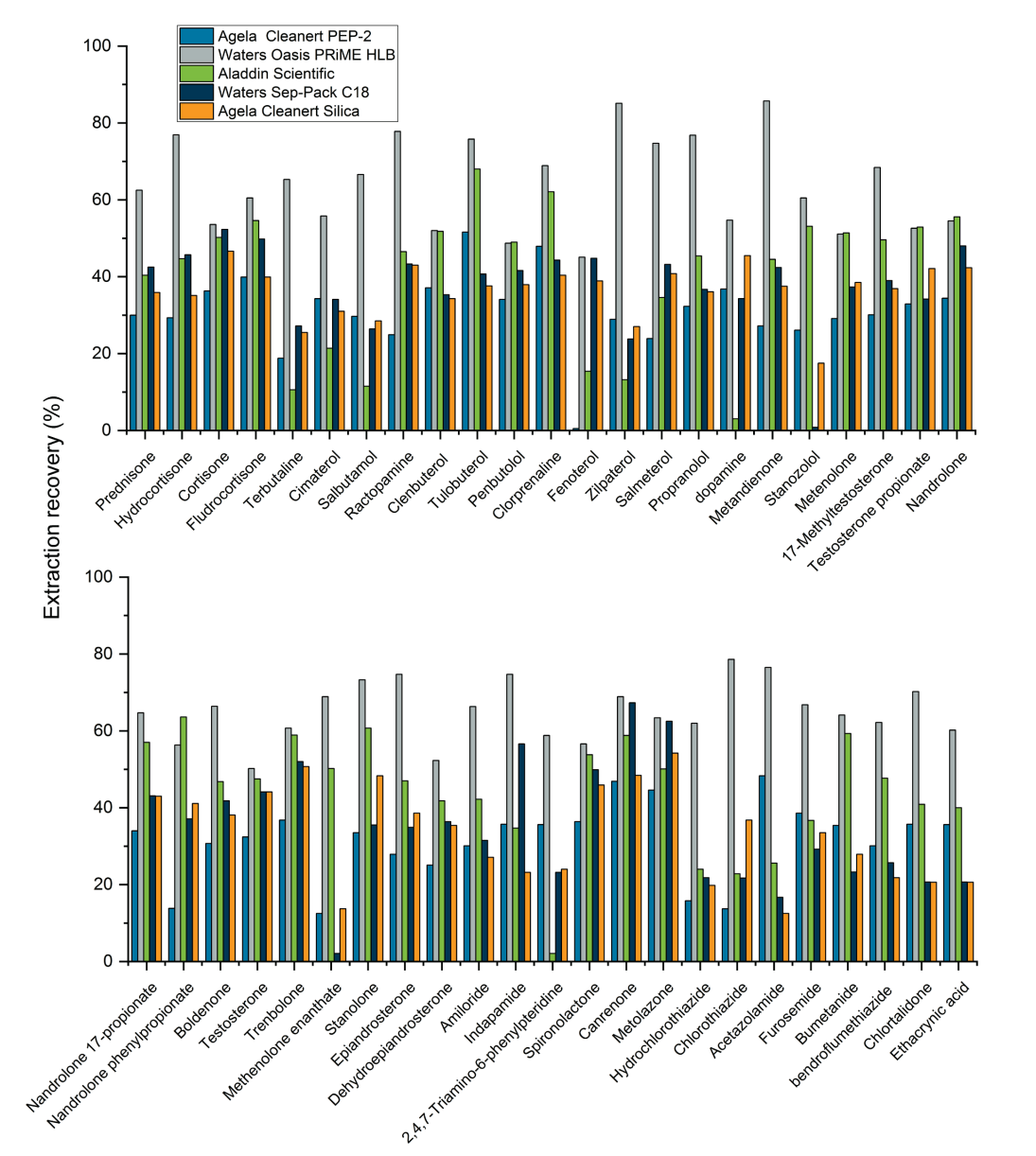 Click to enlarge
Click to enlarge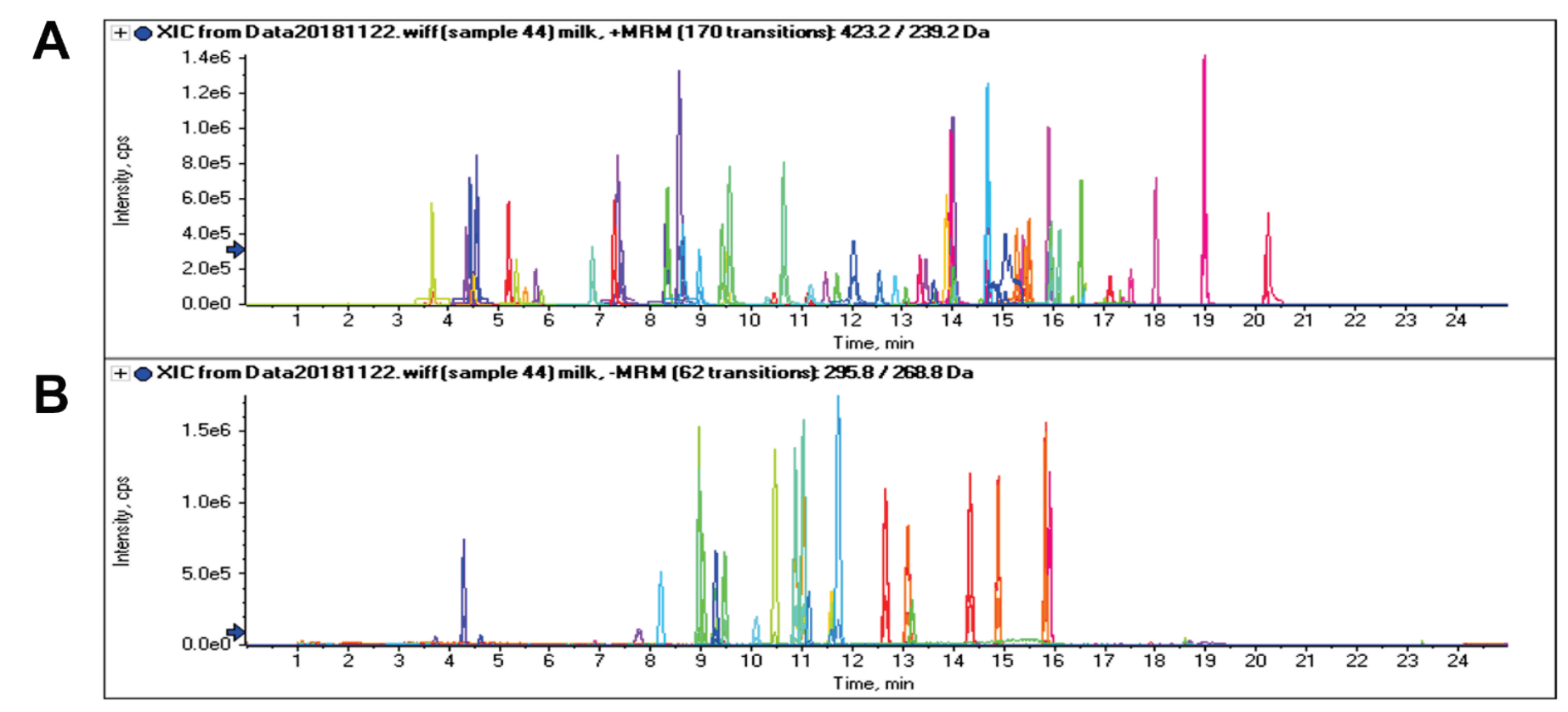 Click to enlarge
Click to enlarge