Overview
Residual nucleic acids require thorough assessment due to potential safety risks. Discover intuitive workflows for analyzing host cell DNA and RNA that provide meaningful answers based on high-quality results, and execute your product development and quality control processes with confidence.
Be empowered to tackle the analytical challenges of plasmids, vaccines and therapeutic proteins derived from bacterial, insect or mammalian cells.
Workflow
Residual DNA analysis
Host cell DNA poses a potential safety risk for cell culture-derived vaccines and therapeutic products. Reliable size determination and simultaneous quantitation of residual DNA are crucial to enable risk assessment and ensure product safety.
Be empowered to address safety concerns linked to residual DNA with high-quality solutions based on capillary gel electrophoresis. Understand your product changes and be confident in determining risks more easily and reproducibly.
-
Determine risk based on the quantity and size of impurities
-
Customize size ranges to your product needs
-
Benefit from an intuitive and highly sensitive solution
-
Increase confidence in DNA assessments with excellent quantitative performance
-
Cover compliance needs through compatibility with the Empower Chromatography Data System (CDS)
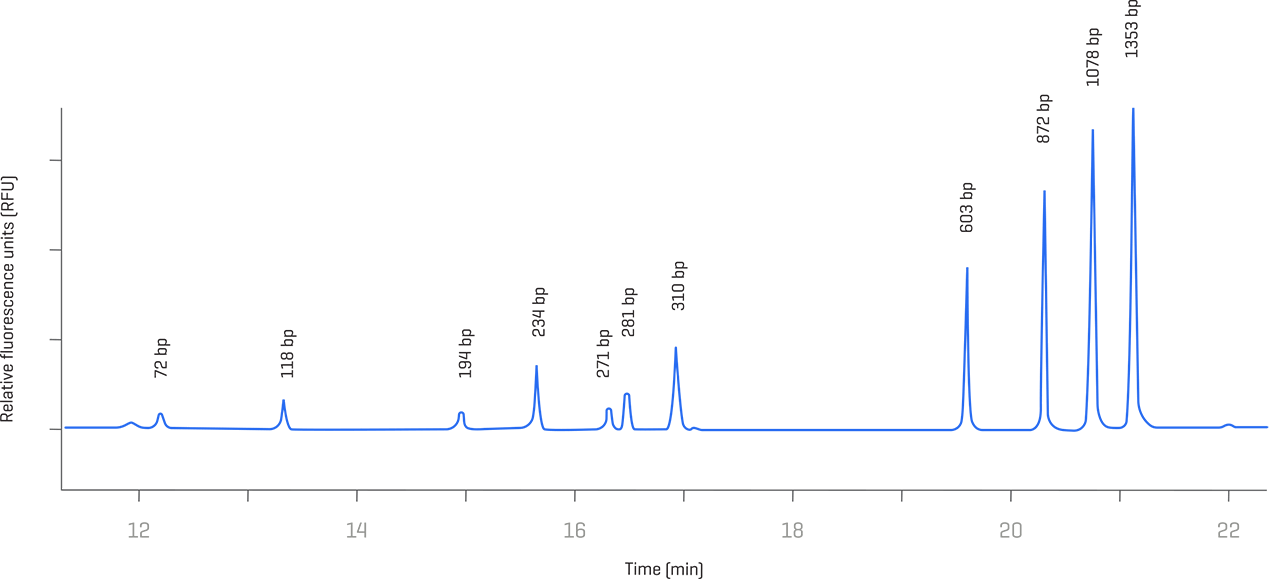
Residual DNA analysis
Solution
Featured resources
Learn from the scientist Dr. Peter Holper about the need for residual nucleic acid analysis, and get insight into the benefits and drawbacks of different analytical techniques.
Explore how the team at MedImmune addressed their challenges around residual host cell DNA and the associated potential safety concerns for cell culture-derived products. Learn how the scientists determined the quantity of residual DNA, assessed the size distribution and evaluated the performance of their optimized method.
Remove barriers to assessing residual nucleic acids and take control of process-related impurities with accurate and reliable workflows. Characterize sizes and quantities of residual host cell DNA for informed decision-making.
Workflow
Residual RNA analysis
Residual host cell RNA can affect product quality and pose risks, such as eliciting undesired immune responses.
Take control of process-related nucleic acid impurities with accurate, reliable and high-quality data. Assess size distribution over a wide range and determine associated quantities with confidence. Be empowered to make informed decisions for risk assessments with turnkey solutions that meet your needs.
-
Determine risk based on the quantity and size of impurities
-
Avoid the sequence bias associated with methods based on polymerase chain reaction (PCR)
-
Benefit from an intuitive and highly sensitive solution
-
Increase confidence in impurity assessments with excellent quantitative performance
-
Cover compliance needs through compatibility with the Empower CDS
Residual RNA analysis
Solution
Featured resources
Reclaim your time by simultaneously assessing viral vector genome integrity and purity with high resolution over an extended size range. Determine impurity quantity and size information for confident decision-making.
Residual RNA analysis
Solution
Featured resources
Learn from the scientist Dr. Peter Holper about the need for residual nucleic acid analysis, and get insight into the benefits and drawbacks of different analytical techniques.
All resources
Learn from the scientist Dr. Peter Holper about the need for residual nucleic acid analysis, and get insight into the benefits and drawbacks of different analytical techniques.
Explore how the team at MedImmune addressed their challenges around residual host cell DNA and the associated potential safety concerns for cell culture-derived products. Learn how the scientists determined the quantity of residual DNA, assessed the size distribution and evaluated the performance of their optimized method.
Remove barriers to assessing residual nucleic acids and take control of process-related impurities with accurate and reliable workflows. Characterize sizes and quantities of residual host cell DNA for informed decision-making.
Take charge of analyzing double-stranded host cell DNA from 100 to 15,000 bp with excellent resolution and sensitivity across the entire size range. Determine product impurities with greater accuracy, repeatability and precision.
Reclaim your time by simultaneously assessing viral vector genome integrity and purity with high resolution over an extended size range. Determine impurity quantity and size information for confident decision-making.